

In a polyatomic molecule, the dipole moment is the vector sum of the dipole moments of the individual bonds. the extent to which the average electron charges are displaced towards one atom. In a diatomic molecule, it is a measure of the polar nature of the bond i.e. Induced dipole: These occur when one molecule with a permanent dipole repels another molecule’s electrons, inducing a dipole moment in that molecule.
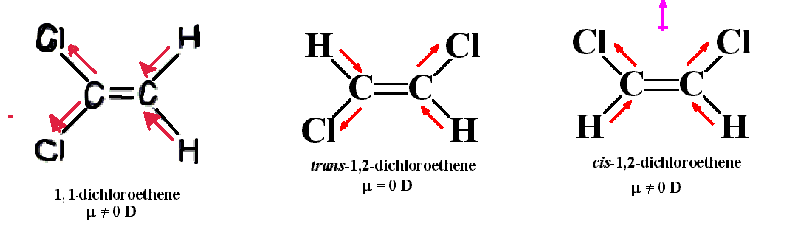
Instantaneous dipoles: These occur due to chance when electrons happen to be more concentrated in one place than another in a molecule, creating a temporary dipole.ģ. Permanent dipoles: These occur when two atoms in a molecule have substantially different electro-negativity with one atom attracting electrons more than another becoming more electronegative, while the other atom becomes more electropositive.Ģ. Dipole moments are often stated in Debyes The SI unit is the coulomb meter. It leads to the development of a positive charge to the H atom and a negative charge to the chlorine atom.Ī molecule having positive and negative charges at either terminal is referred to as electric dipoles or just dipoles. In the case of HCl, the bonding electron pair is not shared equally rather is attracted towards the more electronegative chlorine atom due to its higher electro-negativity which pulls the electrons towards it. Many molecules have dipole moments due to non-uniform distributions of positive and negative charges on their various atoms. The direction of an electric field is defined as the direction of the force on a positive charge, the electric field lines away from a positive charge and toward a negative charge. The direction of the dipole moment corresponds for electric dipoles, to the direction from the negative to the positive charge. Where µ is dipole moment, q is a charge on atom and r is a distance of separation of charge.


 0 kommentar(er)
0 kommentar(er)
